
Camera-based fluorescence microscopy is a fast, reliable technique to capture fluorescent images of cells, spheroids or organoids. The high sensitivity and fast recording time enables high throughput with low phototoxicity and photobleaching at the same time, compared to laser scanning microscopes. A previous drawback was the lower sharpness of the focal plane (Z-axis), known as out-of-focus blur. Leica THUNDER Imagers feature a computational clearing technique which removes this blur in real time, creating confocal-like images of even large sample volumes and helping to visualize more intracellular details.
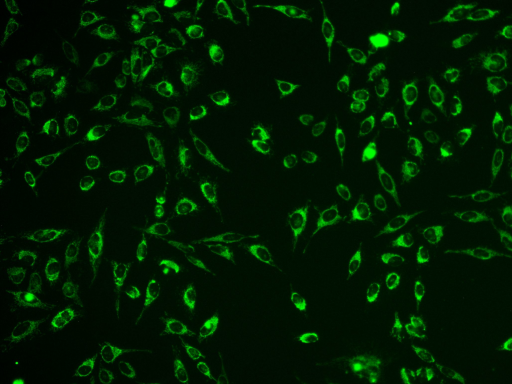
Typical applications are analysis and visualization of cellular kinetics, protein interactions and subcellular localization of proteins using e.g. fluorescent antibodies or fluorescent proteins like the famous GFP and its derivatives, genetically fused to the researcher’s proteins of interest. The equipped laser scanner enables light patterning and photomanipulation of a sample, from large areas down to subcellular compartments. With this method you can control (bio)chemical systems - e.g. to bring a system out of thermodynamic equilibrium (relaxation experiments) to investigate phase separation etc. Other possible applications are optogenetics, light induced (de)activation of proteins, bleaching of fluorescent proteins and uncaging of chemical compounds.
Our Set-up consist of an inverse, computer controlled DMi8 S microscope with a LED8 light source, 2 exchangeable filter cubes with the most common Excitation/Emission wavelengths, a set of objectives ranging from 10x up to 63x Oil and a fully motorized sample stage. Additionally, it is equipped with a laser scanner for light patterning and photomanipulation of a sample (60 mW, 488 nm wavelength).
Leica THUNDER Imager 3D Cell Culture & Infinity Laser Scanner
Laboratory Manager -
Biology
Room:
ZEMOS 1.29
Phone: +49 234 32 -
24740
E-Mail
Physical
Chemistry II
Room:
NC 6/25
Phone: +49 234 32 -
29946
E-Mail